Both Zinc and Tin are relatively low melting point materials with modest strength. Their bonded strength to themselves or other materials is relatively weak compared with the base material they may be bonded to. Galvanized steel bonds are made with nuggets into the steel not the surface zinc. High strengths are obtained. If only the zinc is melted and bonds a very weak joint is formed with the zinc acting as a semi brazing material. Tin coated materials will act the same.
The resistance weldability of copper is very poor. This is due to its high conductivity. Reference materials for the weldability of materials state that resistance welding copper to itself is impractical.
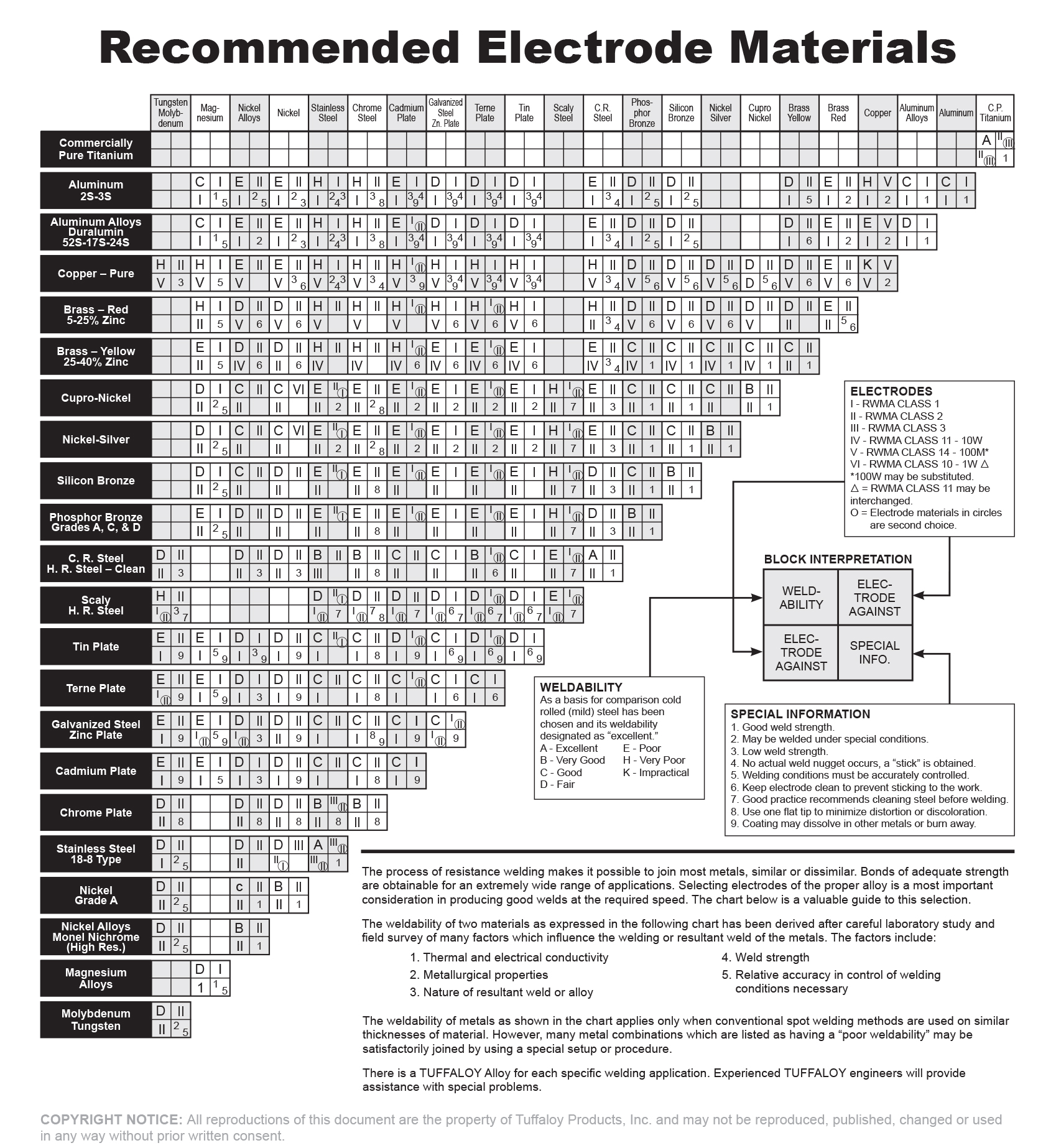
As an alternative a resistance braze may be the only way to get a reliable bond of copper to copper. A coating of zinc or tin is a step in that direction. As stated in the first paragraph they may melt and form a bond to hold the copper members together. Zinc and tin can fail this task because they are not strong and not reliable braze materials. This is addressed in another artricle:
CAN COPPER BE RESISTANCE WELDED?
If better more reliable strengths are desired a true resistance braze using tried and true braze material in the joint would be a better choice to join copper. See the article in this blog:
Reference: RWMA - Resistance Welding Manual 4th Edition
Tuffaloy Resistance Welding Products Catalog - “Recommended Electrode Materials”

