Aluminum has a strong tendency to form aluminum oxide on the surface virtually as fast as it is exposed to air. This is the reason that aluminum can be difficult to spot weld. One must break through this nonconductive aluminum oxide thickness in order to conduct current. Once accomplished there is a weld nugget which in turn will form aluminum oxides. Additionally, there are other unnamed oxides from the copper electrodes on the surface of that nugget. This combination can vary from weld to weld.
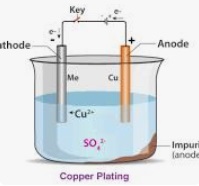
When this is part is electroplated the chemicals in the plating bath will react with the part and weld nugget according to the chemistry it encounters. Some of these nugget chemistries are turning black others are not. Not knowing the chemistry of the plating bath or the part face chemistry no answer can be offered.
A comment must be made that anodized aluminum is made in a plating bath. Have you created an anodizing environment? Partially?
There is an electrochemical reaction present creating the black nuggets vs nonblack nuggets.
COPPER PLATING SKETCH
REFERENCE: RWMA - RWMA Resistance Welding Manual 4th Edition

